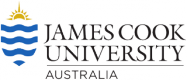Twice the coral trout in Great Barrier Reef protected zones

No-take marine reserves (NTMRs) are widely advocated for conserving exploited fish stocks and biodiversity. Research investigating the effects of the 2004 rezoning of the Great Barrier Reef Marine Park (Emslie et al, 2015) showed that expanding NTMR networks had clear benefits for fishery target, but not non-target, species. A cyclone caused widespread degradation, but target species biomass was retained within NTMRs, with greater recovery potential.
Figure 1. Click on the 4-arrow square to go to the mapping system. Figure 1 shows the location of sites where coral trout were surveyed as part of NERP TE 8.1. The fish symbols are from surveys using BRUVS.
Coral reefs are highly diverse ecosystems that are under increasing pressure from burgeoning human populations worldwide. This has led to calls for strategies to conserve biodiversity, enhance resilience and maintain ecosystem processes. No-take Marine Reserves (NTMRs) are a widely advocated tool for the conservation and management of marine systems (Bohnsack 1993, Halpern & Warner 2002, Claudet et al. 2008) and Australia’s Great Barrier Reef Marine Park (GBRMP) is a world leading example of a multiple-use park that includes several types of NTMR’s, including those closed to commercial and recreational fishing that are known locally as ‘green zones’.
The Great Barrier Reef (GBR) extends for >2000 km along the northeast coast of Australia where development is moderate and human populations are small and centralized. The GBRMP was proclaimed in 1975 under the Great Barrier Reef Marine Park Act 1975 to manage marine resources while allowing commercial and recreational activities to co-exist within its boundaries. There have been three zoning plans since the GBRMP was established. Between 1983 and 1988 a series of regional zoning plans were progressively implemented to protect 1% of the GBRMP from fishing activities. Then from 1989 to 2004, a multiple-use zoning plan was adopted that increased the NTMR area to 4.5% of the GBRMP. Thirdly, following a large-scale planning process from 1999 to 2003, the current zoning plan (Great Barrier Reef Marine Park Zoning Plan 2003) came into effect in July 2004. During this process 70 bioregions were identified within the GBRMP and at least 20% of each bioregion was incorporated into NTMRs. This increased the area of NTMRs to approximately 33% of the GBRMP and included a range of reef and non-reef habitats.
Before the 2004 rezoning of the GBRMP, the Great Barrier Reef Marine Park Authority (GBRMPA) embarked upon a public consultation campaign to explain the rationale behind the re-zoning and to sell the benefits of expanding the area of NTMRs within the GBRMP. The public consultation process led to a loud, vocal response from fishing lobbies stating that extending the amount of NTMRs (or ‘green zones’) would adversely impact the livelihoods of commercial fishers and unfairly exclude recreational fishers from favourite fishing grounds. Such was the uproar that the scientific integrity of many eminent marine scientists was called into question (Scott 2010) especially following the publication of a review paper on the effects of the GBRMP re-zoning (McCook et al 2010). Given this climate of scepticism with accusations of impropriety by Australia’s marine scientists and the management agency, it was important to impartially evaluate the effectiveness of the 2004 re-zoning of the GBRMP against its stated aims.
The establishment of NTMRs has had clear benefits for the primary target of the hook and line fishery, the coral trout (Plectropomus spp; Figure 1). Before establishment of the GBRMP in the 1980s, GBR-wide coral trout biomass was ~5 kg 1000 m-2, but then declined to 1 – 2 kg 1000 m-2 by 1996. Biomass then accumulated on NTMR reefs until the re-zoning in 2004, after which GBR-wide biomass increased rapidly and reached peak levels, similar to or greater than the 1980s levels. On reefs open to fishing biomass increased but at a slower rate than NTMRs (Figure 2) which resulted in an average of ~2.5 times greater coral trout biomass on NTMR reefs than on those open to fishing (Figure 2). Following the re-zoning of the GBRMP in 2004 there was a greater proportion of coral trout larger than the minimum legal size (38cm) inside NTMRs than fished reefs, meaning that coral trout were larger in NTMRs compared to fished reefs (Figure 3).
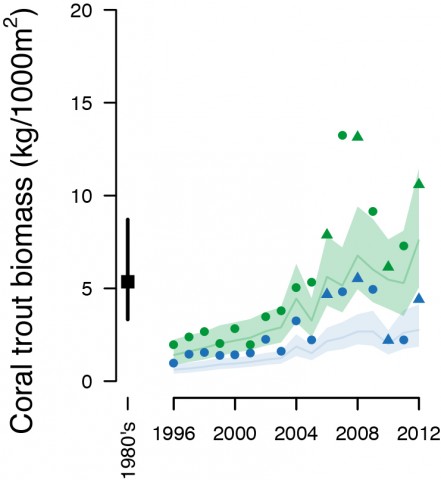
Similarly there was a greater density of coral trout in NTMRs compared to fished reefs (Figure 3), and the size difference combined with a higher coral trout density translated into an 82% higher biomass of coral trout inside NTMRs (Figure 4). Benefits to secondary target fishes (those not directly targeted by fishers but retained if caught) were inconclusive, with secondary target fishes 1% larger and biomass was 30% higher on NTMR reefs compared with fished reefs.
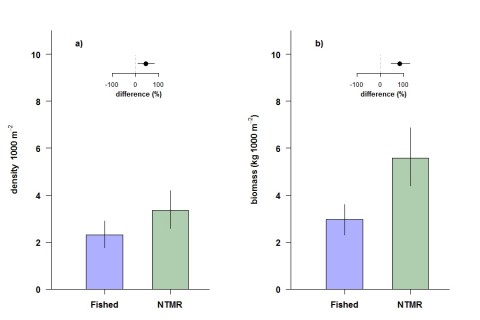
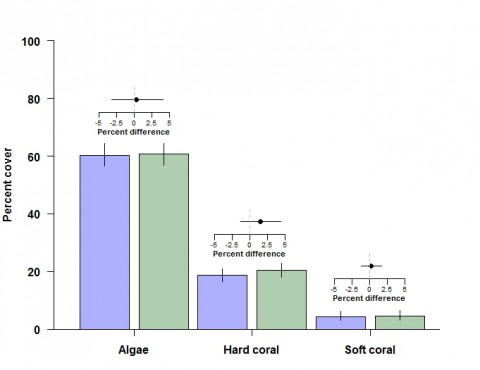
Among other groups of fishes not targeted by the fisheries, detritivores, omnivorous damselfishes and benthic foragers were all between 13% and 35% more abundant in offshore NTMRs compared with fished reefs. Overall species richness of reef fishes was 8% greater inside NTMRs than on fished reefs. There was little evidence that NTMR status exerted any influence on overall community of fishes or benthic organisms, with NTMR zoning status accounting for <1% of the total variation in reef fish and benthic community structure (Figure 4). There were no differences in either inshore or offshore cover of hard coral, soft coral or algae (Figure 5) between NTMR and fished reefs.
Overall, NTMRs have had positive effects on fishery-targeted coral trout populations of the GBR. While our data show these benefits generally accrue over decadal time scales, differences in the density and biomass of coral trout on some NTMR and non-NTMR reefs appeared rapidly. A sharp reduction in fishing effort and mortality may have allowed coral trout to accumulate rapidly inside reserve areas. Some individuals may have migrated to shallower reef areas from deeper refuge areas that were not heavily targeted by fishers (Russ et al. 2008). In addition, long-term protection from fishing facilitated a gradual increase in the mean size of coral trout and the build-up of spawning stock biomass which could provide spill-over and recruitment subsidies to adjacent fished reefs (Francini-Filho & Moura 2008, Almany et al 2009, Harrison et al. 2012). Importantly, the designation of additional NTMR areas in 2004 did not lead to a decline in coral trout biomass on reefs that remained open to fishing because of displacement and concentration of fishing effort, the so-called ‘squeeze effect’ (Halpern et al. 2004, Buxton et al. 2014).While differences in the density and biomass of target species are readily apparent, fishers on the GBR target only a few species. NTMRs on the GBR provide little benefit for non-target species, for biodiversity, or the structure of fish and benthic assemblages because they are not affected by current levels of fishing. In contrast to recent work in the Caribbean (Mumby & Harborne 2010), the lack of difference in the diversity or assemblage structure of reef fish and benthic communities between NTMRs and fished reefs suggests that coral reef assemblages of the GBR appear to be largely structured by bottom up processes rather than top-down effects of fishing. Top-down control of GBR fish assemblages through fishing appears unlikely as there is relatively light fishing pressure that only targets a small number of generalist predatory fishes that consume diverse and abundant prey.
References
Almany, G. R., Connolly, S. R., Heath, D. D., Hogan, J. D., Jones, G. P., McCook, L.J., Mills, M., Pressey, R. L. and Williamson, D. H. (2009). Connectivity, biodiversity conservation and the design of marine reserve networks for coral reefs. Coral Reefs 28, 339-351.
Babcock, R. C., Shears, N. T., Alcala, A. C. , Barrett, N. S., Edgar, G. J., Lafferty, K. D., McClanahan, T. R., and Russ, G. R. (2010). Decadal trends in marine reserves reveal differential rates of change in direct and indirect effects. Proc. Natl. Acad. Sci. 107, 18256-18261.
Bohnsack, J. A. (1993). Marine reserves: They enhance fisheries, reduce conflicts and protect resources. Oceanus 36, 63-71.
Claudet, J., Osenberg, C. W., Benedetti-Cecchi, L., Domenici, P., Garcıa-Charton, J.-A., Perez-Ruzafa, A., Badalamenti, F., Bayle-Sempere, J., Brito, A., Bulleri, F., et al. (2008). Marine reserves: Size and age do matter. Ecol. Lett. 11, 481-489.
Emslie Michael J., Murray Logan, David H. Williamson, Anthony M. Ayling,. Aaron MacNeil, Daniela Ceccarelli, Alistair J. Cheal, Richard D. Evans, Kerryn A. Johns, Michelle J. Jonker, Ian R. Miller, Kate Osborne, Garry R. Russ, and Hugh P.A. Sweatman. (2015) Expectations and Outcomes of Reserve Network Performance following Re-zoning of the Great Barrier Reef Marine Park Current Biology 25, 1–10 https://dx.doi.org/10.1016/j.cub.2015.01.073
Francini-Filho, R. B., and Moura, R. L. (2008). Evidence for spillover of reef fishes from a no-take marine reserve: An evaluation using the before-after control-impact (BACI) approach. Fish. Res. 93, 346-356.
Halpern, B. S., and Warner, R. R. (2002). Marine reserves have rapid and lasting effects. Ecol. Lett. 5, 361-366.
Halpern B. S., Gaines, S. D., and Warner, R. R. (2004). Confounding effects of the export of production and the displacement of fishing effort from marine reserves. Ecol. Appl. 14, 1248–1256.
Harrison, H. B., Williamson, D. H., Evans, R. D., Almany, G. R., Thorrold, S. R., Russ, G. R., Feldheim, K. A., van Herwerden, L., Planes, S. , Srinivasan, M., et al. (2012). Larval export from marine reserves and the recruitment benefit for fish and fisheries. Curr. Biol. 22, 1023-1028.
McClanahan, T. R., Graham, N. A. J., Calnan, J. M., and MacNeil, M. A. (2007). Toward pristine biomass: reef fish recovery in coral reef marine protected areas in Kenya. Ecol. Appl. 17,1055-1067.
McCook, L.J., Ayling, T., Cappo, M., Choat, J.H., Evans, R.D., De Freitas, D.M., Heupel, M., Hughes, T.P., Jones, G.P., Mapstone, B., Marsh, H., Mills, M., Molloy, F.J., Pitcher, C.R., Pressey, R.L., Russ, G.R., Sutton, S., Sweatman, H., Tobin, R., Wachenfeld, D.R., and Williamson, D.H. (2010) Adaptive management of the Great Barrier Reef: A globally significant demonstration of the benefits of networks of marine reserves. PNAS 107: 18278-18285.
Mumby, P. J., and Harborne, A. R. (2010). Marine reserves enhance the recovery of corals on Caribbean reefs. PLoS ONE 5(1), e8657.
Russ, G. R., Cheal, A. J., Dolman, A. M., Emslie, M. J., Evans, R. D., Miller, I., Sweatman, H., and Williamson, D. H. (2008). Rapid increase in fish numbers follows creation of world’s largest marine reserve network. Curr. Biol. 18, R514-R515.
Scott, P. (2010) Reefgate – or a spat between Scientists? Go Boating Magazine May 2010: 18 – 24.